Low Ir-OER Catalysts
Background
As the share of electricity produced from renewable energies is rising, it becomes more and more necessary to find a way to store excess energy and to couple electricity to heat and mobility. The production of hydrogen is the most promising way to achieve these goals and become independent of fossil energy. Hydrogen can be produced via electrolysis of water with electricity in electrochemical cells. These cells differ in their electrolyte, operation temperature and half-cell reactions. The most prominent are the alkaline water electrolysis cell (AWE), the proton-exchange-membrane water electrolysis cell (PEMWE) and the solid oxide water electrolysis cell (SOWE).[1]
The PEMWE features a compact design, high current and voltage densities, fast start and stop behaviour and high gas purities. Inside a PEMWE, water is oxidized at the anode to oxygen, the emerging protons are conducted through the polymer membrane (e.g. Nafion®) and reduced to hydrogen at the cathode. Hereby, the oxygen evolution reaction (OER) at the anode is the reaction with the slowest kinetics. The typical state-of-the-art catalyst used for the OER in acidic media is IrO2.
However, the issue with iridium is the price and the availability. Iridium is one of the rarest metals (even rarer than platinum) and therefore catalysts become expensive. Nevertheless, there is no known alternative to Iridium as catalyst for the OER in acidic medium, so the aim is to enhance the reactivity and stability while reducing the Iridium content.[2]
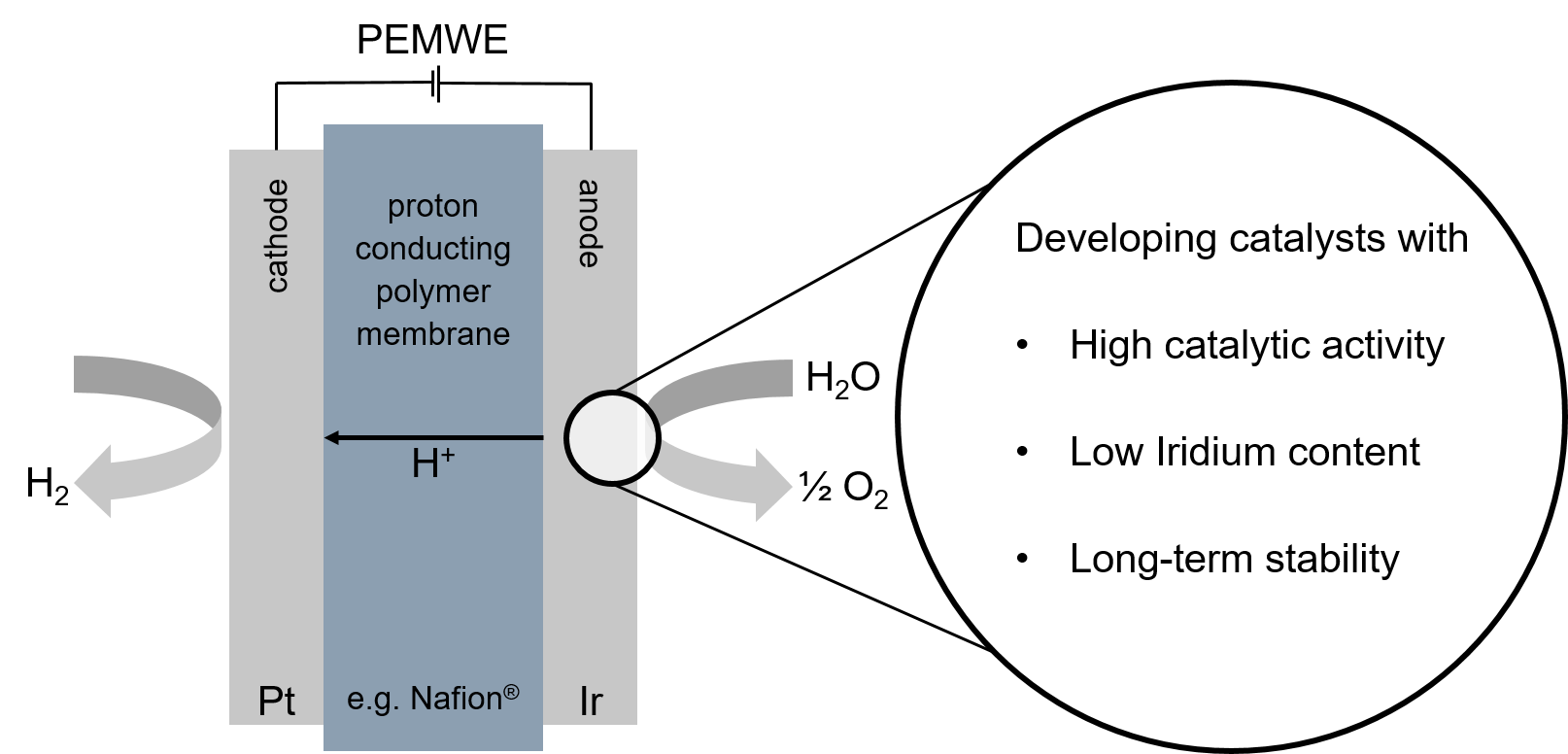
Research focus
The focus of this project is to develop a catalyst, which contains significantly less iridium than the IrO2 benchmark catalyst, shows excellent performance and reactivity and is stable even under enhanced temperatures in a high temperature PEMWE. This goal could be achieved through embedding Ir in properly tailored oxide structures. Through interactions with the surrounding atoms the properties of the Iridium sites are fine-tuned to enhance the overall catalytic activity.
Framework program
This project is part of the HOPLYT-project, funded by the Federal Ministry of Education and Research. Hereby, the FAU cooperates with the Leibniz University Hannover, the University of Rostock, the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy (HI-ERN) and the company Riva power systems to produce a PEMWE that could be used for elevated temperatures.
Funding by:
References
[1] M. Carmo, D. L. Fritz, J. Mergel, D. Stolten, International Journal of Hydrogen Energy 2013, 38, 4901-4934.
[2] T. Reier, H. N. Nong, D. Teschner, R. Schlögl, P. Strasser, Advanced Energy Materials 2017, 7.
