Background
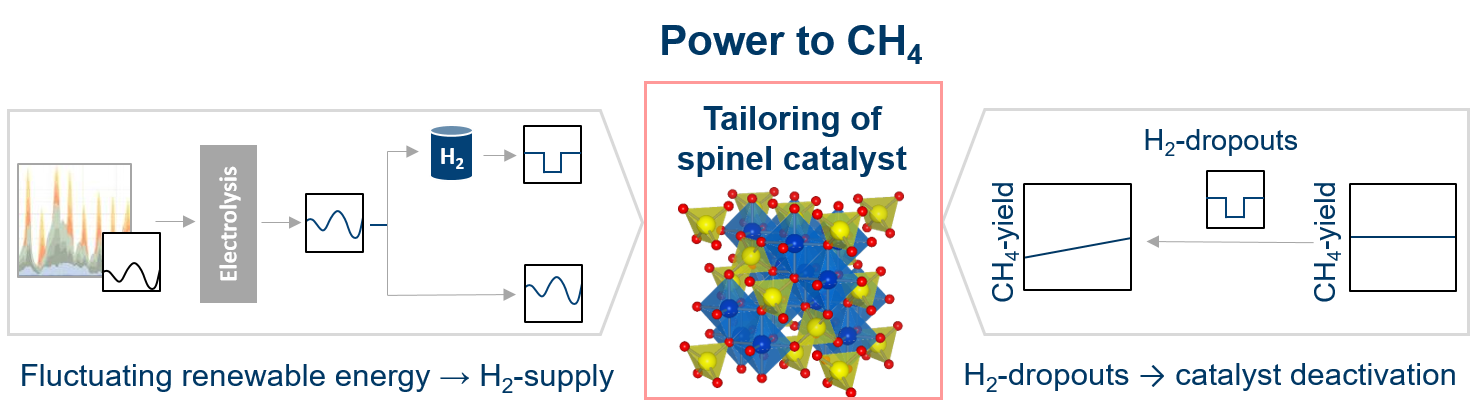
One of the biggest challenges facing society today is to curb man-made climate change caused by the continued burning of fossil fuels and the associated emissions of greenhouse gases such as CO2. Electrification of society is an important step in this process, and the use or storage of surplus electricity generated by fluctuations in renewable energy sources is a major challenge. So-called power-to-X technologies represent a promising approach here. To ensure the economic viability of such a process, an active, selective and stable catalyst is required. CO2 methanation is used as model reaction to evaluate the catalytic performance and stability under static and dynamic reaction conditions
In CO2 methanation, spinel phases are often considered as undesirable leading to deactivation. However, they can also be considered as well-dispersed solid solutions suitable as catalyst precursors leading to the formation of highly active and finely dispersed active sites. In addition, spinels offer the possibility of reversing potential catalyst deactivation due to their redox reversibility.
Due to the use of renewable energy for hydrogen production and the associated hydrogen dropouts, these dynamic operating conditions can be utilized to reactivate the catalyst, which is summarized in the so-called concept of the „Dynamic Responsive Methanation Catalysts“ (see Figure 1). In contrast, commonly used supported catalysts (e.g. Ni/Al2O3). are prone to irreversible deactivation under the dynamic hydrogen dropouts conditions. [1]
References
[1] B. Mutz, A. M. Gänzler, M. Nachtegaal, O. Müller, R. Frahm, W. Kleist, J.-D. Grunwaldt, Catalysts 2017, 7, 279.
This project is conducted in cooperation with TU Dortmund and KIT in the framework of the DFG Priority Programme SPP2080 “DynaKat” (www.spp2080.org). We kindly acknowledge funding by the DFG.



Project related Publications:
- , , , :
Modifying Spinel Precursors for Highly Active and Stable Ni‐based CO2 Methanation Catalysts
In: ChemCatChem (2022)
ISSN: 1867-3880
DOI: 10.1002/cctc.202200563
- , , , :
- , , , :
Solid solutions in reductive environment – A case study on improved CO2 hydrogenation to methane on cobalt based catalysts derived from ternary mixed metal oxides by modified reducibility
In: Journal of Catalysis 382 (2020), S. 385-394
ISSN: 0021-9517
DOI: 10.1016/j.jcat.2019.12.045
- , , , :
